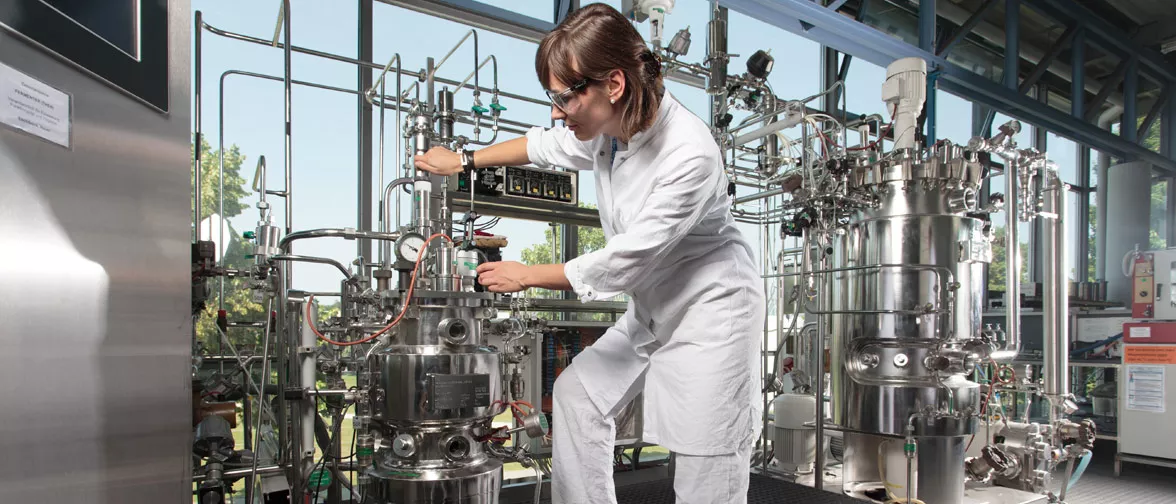
Pharmaceutical Bioprocess Engineering
Bachelor of Science (B.Sc.)
Does the idea of contributing to people's health inspire you? How about changing the pharmaceutical industry with innovative processes, or combining natural sciences and engineering? With the Pharmaceutical Bioprocess Engineering program, which is unique in Germany, you will not only learn to be an outstanding process engineer, you will also gain the basic biotechnological knowledge required to analyze and improve molecular and cellular processes, process active biotechnological substances into drugs, optimize production methods, and develop new concepts in plant engineering. Redesign the biotechnological, chemical, and pharmaceutical industry – with a Bioprocess Engineering program at the TUM!
Key Data
| Type of Study | Full Time |
| Standard Duration of Studies | 6 Semesters |
| Credits | 180 ECTS |
| Start of Degree Program | Winter Semester |
| Application Period | May 15 - September 15 |
| Admission Category | Unrestricted Admission |
| Language of Instruction | German |
| Main Location | Weihenstephan (Freising) |
| Costs |
The Bachelor's program in Pharmaceutical Bioprocess Engineering combines the natural sciences, engineering and economics and thus opens up a broad spectrum of roles. Knowledge from biology and biochemistry is increasingly being used for industrial production processes. Nature's capacity for synthesis is endless. Make use of this potential and develop innovative processes that are gentler on products and more environmentally friendly than chemical processes. In addition, many active substances of the human body, such as insulin, cannot be produced chemically, but only biotechnologically.
As a future expert, you will not only be distinguished by your knowledge in the fields of biotechnology, process engineering, and process automation but above all by your understanding of systems to meet the special requirements of drug production. So join us in the development of microorganisms that are used to produce biotechnological products, learn analytical methods for process monitoring and design concepts for new production plants.
Pharmaceutical bioprocess technology is a young, innovative key technology of the 21st century. Take advantage of the potential growth and the increasing economic importance of this industry for your future.
Research and develop new manufacturing processes, monitor the quality of medicines, plan production facilities, or be responsible for organizing the operating technology – your possible fields of activity are diverse. As a bioprocess technician, you will find jobs in these areas, particularly in the biotechnological, chemical, cosmetic and pharmaceutical industries, as well as in plant engineering.
Still not had enough of studying? The majority of students continue their studies after their Bachelor's program with a Master's program in Pharmaceutical Bioprocess Engineering.
At the beginning of the course of study, the focus is on the basics of natural sciences, covering the areas of biology, chemistry, mathematics, and physics. Later on, biology and chemistry will educate you in the fields of microbiology and biochemistry. Physics and mathematics form the foundation for a specialization in the engineering sciences.
From the 3rd semester onwards, you will begin to specialize: In modules such as Pharmacology and Toxicology or Molecular Biotechnology you will learn about the mechanisms and effects of drugs. You will then link this knowledge with areas such as bioprocess engineering or packaging technology since you know that technical processes can also affect biological systems. To be able to adapt processes or plants accordingly, you will need expertise in the fields of technical mechanics, fluid mechanics, and apparatus engineering. The laboratory internships will allow you to investigate various questions for yourself.
In the elective area, you have the opportunity to build your profile according to your interests and to follow your talents.
At the end of your studies, you will immerse yourself in your first scientific work and write your Bachelor's thesis.
A further part of your studies is the 6 weeks of work experience, during which you can gain a first insight into the professional field of bioprocess technicians.
Interested? Just take a look at an exemplary curriculum!
The course focuses on pharmaceutical bioprocess technology on the one hand and engineering sciences on the other. Both areas are continuously enhanced and interlinked during the course – biotechnological matter is viewed in the context of real processes. After your studies, you will be able to design biotechnological plants, monitor production processes, ensure the quality of products, produce and analyze biotechnological products, and produce common pharmaceutical substances. Besides, you will be able to work safely and scientifically correctly in the laboratory, know pharmaceutical technologies, and be able to take measures for product safety.
Nobody will be able to mislead you at any level of pharmaceutical bioprocess technology after your studies.
The TUM website on the application process provides a general overview. The following sections contain information specific to this degree program.
Application Requirements and Eligibility
Entrance Qualification
A higher education entrance qualification is the prerequisite for applying for a place in this degree program. This can be either a relevant school-leaving certificate or proof of sufficient professional qualifications.
Admission Prerequisites
The admission to this degree program is unrestricted.
Verification of Language Skills
The language of instruction in this degree program is German. Therefore, it is necessary to prove sufficient German language skills with your application. Please check whether you need a language certificate and which types are accepted.
Preliminary Documentation (VPD)
Applicants who have obtained their higher education entrance qualification not at a German school require a preliminary documentation (VPD). First, you must apply for the VPD at uni-assist, and second, upload it with your application to your application account (see section “Online application” below).
Please find further critical information on the TUM website for international applicants.
| Important |
|---|
| The processing of the VPD at uni-assist can take 6-8 weeks! We therefore recommend an early application; otherwise, a delay in processing the application documents at TUM has to be expected since only complete application documents can be processed. |
Application Period and Start of Program
The application period is from 15 May to 15 September each year for the following winter semester. We strongly recommend that international students apply as early as possible. You can start this program only in the winter semester. Please find the semester dates on the TUM website.
Semester fees and tuition fees at TUM
All students pay a semester fee for the Studierendenwerk.
| Tuition fees for international students |
|---|
| Tuition fees are charged for international students from countries outside the European Economic Area. All information on fees, waivers, and scholarship programs can be found on the website on tuition fees for international students. |
Online Application
You can only apply online via our application portal in TUMonline. There, you set up an applicant account (manual). Please upload all required documents to your applicant account by the end of the application period. Documents issued in languages other than German or English must be translated into German or English by a sworn translator. Both the original document and its translation must be uploaded as one PDF file.
| Important |
|---|
| We can only consider your application if you have submitted all required documents completely and within the application deadline. Documents submitted by e-mail cannot be processed. |
Enrollment
As soon as you have received a letter of admission for this degree program, you accept your study place in your applicant account. You then upload the remaining documents for enrollment and pay the semester fees. All relevant steps will be displayed in your TUMonline account. Additionally, you can learn the details of the enrollment procedure.
| Please note |
|---|
| From admission on, we will only contact you via your tum.de or mytum.de e-mail address, which you can find in your TUMonline account. |
Questions
If you have any questions about the application process, please contact the student advisor (see end of this page).
Suitable Master's programs at the TUM School of Life Sciences
- Brewing and Beverage Technology M.Sc.
- Food Technology M.Sc.
- Nutrition and Biomedicine M.Sc.
- Pharmaceutical Bioprocess Engineering M.Sc.
Other degree programs that might also interest you:
- Degree Program Documentation
The degree program documentation presents the concept of the study program.
- Module Catalog
The module catalog lists all modules of the current version of the degree program, and is updated before the start of each semester.
Enrolled students who are studying in previous degree program versions can find their module catalog in TUMonline.
Tip: For completed modules, you can create an individual module catalog in the TUMonline application “My Studies” (icon top right). TUM School of Life Sciences recommends to use this option every semester (at the latest after graduation), as it facilitates the recognition of modules and achievements in your future professional life!
- Curriculum
The degree chart gives you an overview and recommendation, which modules you should take in which semester according to academic and examination regulations (FPSO).
- Timetable (TUM ID required for login):
This timetable is intended to give you an overview of the planned compulsory and elective modules of a semester. It is for orientation purposes only and will not be updated during the semester!
Via TUMonline you can have a timetable created for each semester according to FSPO.
You can also create your own individual timetable, which can contain not only the dates of the selected courses, but also your personal appointments.
First steps with TUMonline
- General Academic and Examination Regulations at TUM (APSO)
- Academic and Examination Regulations (FPSO)
Examination dates & registration via TUMonline
In TUMonline you can register for the module examinations that accompany your studies.Important: You can only take most of the exams if you have actively registered yourself via TUMonline within the registration period. The registration and deregistration period will be displayed at the exam date.
Please note! In the basic and orientation exams (GOP) in the 1st and 2nd bachelor semester you will automatically be registered for all examinations during the respective semester, i.e. independent registration is not necessary in the 1st academic year.- Further examination matters
- Board of Examination
The board of examination is a committee consisting of university professors and lecturers of the degree program. The chairperson of the board of examination of your degree program is Prof. Dr.-Ing. Heiko Briesen. Applications to the board of examination are received by the secretary and submitted to the board of examination for decision. You can reach the secretary by sending an email to examination.co(at)ls.tum.de.
Detailed, degree program - specific information about graduation can be found in the
Wiki Life Sciences – Study and Teaching - Graduation (TUM ID required for login)
Personal student advising

Dr. Paula Singmann
Campus Office
ground floor, room 08
Tel. +49 8161 71 4362
brew-food-bpt.co(at)ls.tum.de
Consultation hours:
Presence: Tuesdays, 2:30 - 3:30 p.m.
Telephone: Thursdays, 2:30 - 3:30 p.m.
and by appointment
Everything you need to know!
Many general questions can already be answered by the FAQs.
Current and course-specific information for students of the TUM School of Life Sciences
Wiki Study and Teaching (with TUM login only)